
REVIEW
SA JOURNAL OF DIABETES & VASCULAR DISEASE
54
VOLUME 12 NUMBER 2 • NOVEMBER 2015
Diabetic patients, more than any other subset, show the
greatest difference in telomere length compared to non-diabetics.
26
Type 2 diabetes is considered a cardiovascular risk equivalent.
27,28
It is postulated that telomere shortening induces pancreatic
β
-cell
senescence. Like atherosclerosis, diabetes is thought to be a
premature-ageing syndrome.
26
The study of telomeres may therefore provide in a single marker,
the combined influence of genetics, environmental risk and ageing
in predicting risk and identifying susceptible individuals prone to
developing coronary artery disease. This is especially relevant in our
community, which has a high incidence of both premature coronary
artery disease and type 2 diabetes.
29,30
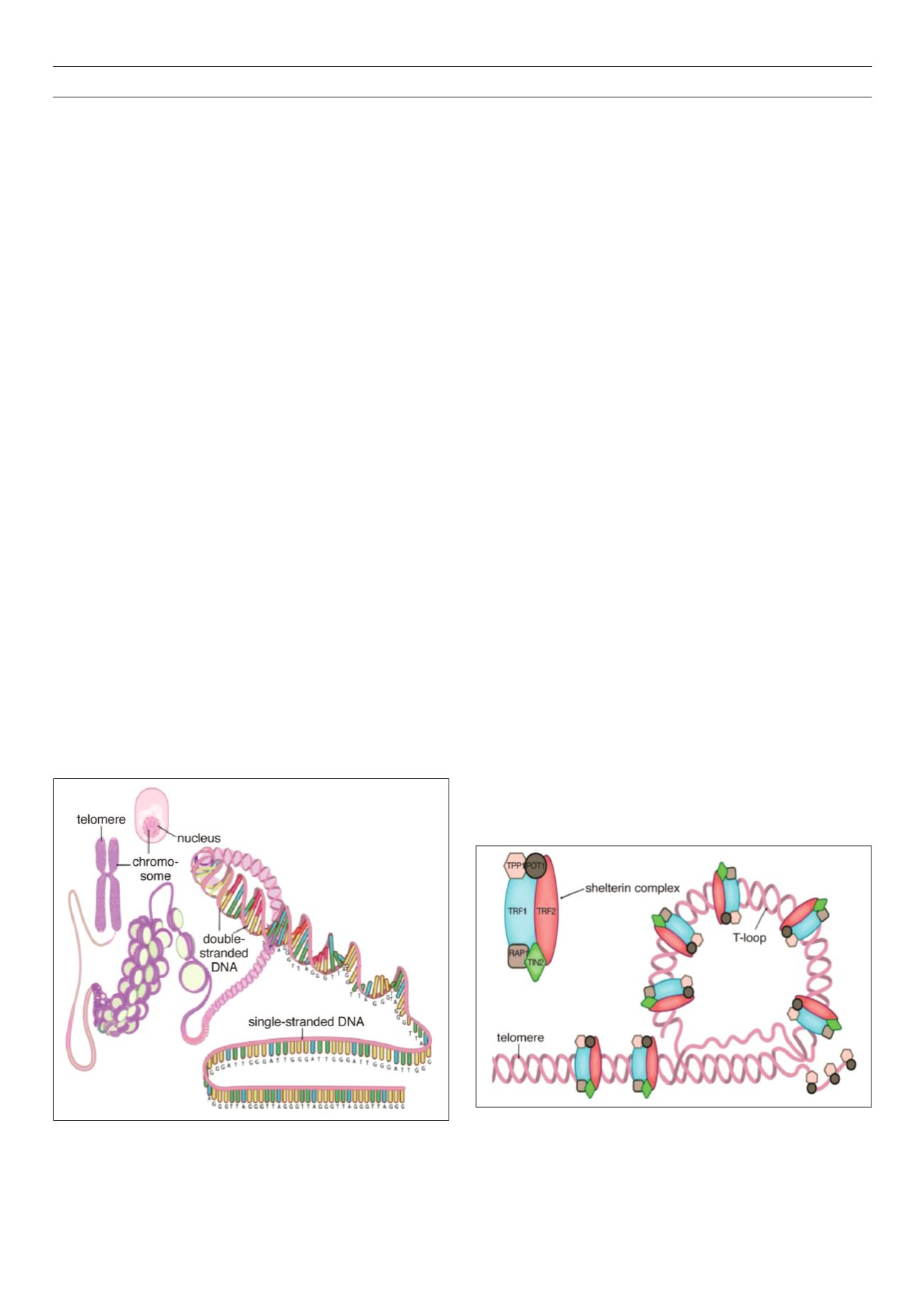
Structure and function of the telomere complex
Telomeres have a dynamic structure that is thought to switch
between a closed, protected state and an open, extendable
state, which allows the DNA terminus to undergo replication.
The protected state is necessary for safeguarding the integrity of
genomic material, whereas the extendable state allows the enzyme
telomerase to extend short telomeres (Figs 1, 2).
31
Telomere components include:
• The DNA component: this consists of tandem repeats of the
hexanucleotide 5’-TTAGGG-3’ (T = thymine, A = adenine, G =
guanine) and has a high guanine content. The bulk of telomeric
DNA is arranged in the double-stranded configuration, which
then ends in a single-stranded extension. The single-stranded
overhang folds back to form a terminal loop, which prevents
the end of the telomere from being recognised as a damaged,
broken end. Telomere shortening is thought to destabilise this
loop.
8,14,31
• Shelterin proteins: these proteins bind and protect the loop
structure and are termed shelterin because they shelter the
chromosome end.
32
An inability to form the terminal loop will
leave the chromosome ends uncapped, resembling a DNA
break and provoking DNA repair mechanisms. The shelterin
complex consists of six proteins, which have specific functions
in telomere replication and end protection.
The six proteins are: TRF1 and TRF2: telomere repeat binding
factors 1 and 2, which are the two major proteins; POT1: protection
of telomeres 1; TPP1: tripeptidyl peptidase 1; TIN2: TRF1-interacting
protein 2; and RAP1: repressor activator protein 1. Whereas the
shelterin proteins are a constant fixture at the telomere end, other
accessory proteins are intermittently recruited to the telomere.
These proteins include the tankyrases tank 1 and 2, Ku 70/86 and
poly-ADP ribose polymerase-1 (PARP-1), which influence the control
of telomere length and repress the DNA damage response.
31,33,34
• The CST complex: an additional telomere-associated complex,
known as the CST, has recently been identified. It binds single-
stranded DNA and appears important for both telomere
protection and replication.
31
• Telomerase: in order for cellular repair to take place as well
as for species survival, stem cells and reproductive cells need
to be able to proliferate without the penalty of progressive
telomere shortening.
31
These cells, unlike somatic cells, contain
the enzyme telomerase, which is capable of adding DNA
sequences to the chromosome terminus to compensate for the
loss sustained during replication. Telomerase is made of Terc –
the RNA component that serves as a template for the synthesis
of new telomeric DNA, and TERT – a reverse transcriptase which
is the catalytic subunit representing the rate-limiting step in
telomerase activity.
12,14,33,35
A variety of accessory proteins have
important roles in telomerase biogenesis and localisation.
Telomere homeostasis
Telomere length in proliferating cells is influenced by the
following factors.
• Factors that shorten telomeres:
– telomere attrition during cell division
– DNA damage due to oxidative stress caused by environmental
risk factors
– specific exonucleases involved in the degradation of RNA
primers used for DNA replication
– deficiency of Rad 54, which is involved in DNA repair
– histones: methylation of histones H3 and H4 diminishes
telomerase activity.
36
• Factors that maintain telomere length:
– Telomerase: in addition to the level of telomerase within a
cell, telomere length is also dependent on the delivery of
Figure 1
. A simplified scheme depicting the structure of the telomere and its
location on the chromosome in the cell. Reproduced with permission.
126
Figure 2
. Scheme showing the terminal end of the telomere concealing the
terminal single-stranded part with the help of the shelterin complex. Reproduced
with permission.
126



















